News:
As of November 1, 2019, we have started accepting requests for cancer genome medicine covered by national health insurance.
OncoPrime and Guardant360 treatments, which are not covered by national health insurance, also remain available.
At the Toyama University Hospital Cancer Genome Medicine Promotion Center, we offer gene panel tests for patients interested in requesting cancer genome medicine.
Before requesting a gene panel test, we ask that you first read this page and make sure you understand what is written here, then consult your doctor at your current hospital or medical department. That medical facility can then make an appointment at the Cancer Genome Medicine Promotion Center.
Final decisions regarding applications are made based on a consultation with the Cancer Genome Medicine Promotion Center, and the results of close discussion with the doctor in charge.
What Is Genome Medicine?
Part 1: Cancer Genome Medicine
1.1 What Is Genome Medicine?
1.2 Fundamental Genome Knowledge
1.3 Fundamental Cancer Knowledge
Part 2: Genetic Testing
2.0 Who Is Genetic Testing For?
2.1 Gene Panel Testing
2.2 What Can Be Learned through Gene Panel Testing
2.3 The Samples Used for the Test
Part 3: Requesting Cancer Genome Medicine
3.1 Requesting Genome Medicine through Your Current Medical Institution
3.2 Requesting Genome Medicine through Toyama University Hospital
Part 4: Costs
Specifics of the Test
Gene Panel Tests Available at Toyama University Hospital
Gene Panel Test Precautions
Gene Panel Test Costs
Genome medicine is a form of precision medicine based on a single individual’s genome data, optimized for that patient’s constitution and the condition of their disease. More specifically, in addition to conventional tests like blood sampling or imaging tests, genome medicine uses genome tests with well-established quality and reliability, to provide patients with the most effective therapy and prevention, as well as predictions of disease onset. In modern medicine, diseases are diagnosed based on the results of various clinical tests, and treatment is based on the guidelines for the disease. In other words, ordinarily, every patient diagnosed with a given disease receives the same “one size fits all” treatment and medication as any other patient.
But of course, effects and side effects vary from person to person — a given treatment might work on some people but not on others, or might cause complications, or even have serious side effects that could lead to death.
By analyzing the genome of a single patient’s cancer and selecting the optimal therapy or anti-cancer drugs, cancer genome medicine promises to maximize the effectiveness of therapies while minimizing their side effects, providing optimized treatment based on each patient’s clinical condition.
What Are Genes?
Cells are the fundamental building blocks of the human body, and genes serve as the blueprints to synthesize the proteins that form the basic structure of cells. These genes are passed down from parent to child; this is known as “heredity.” These genes are stored as DNA, which is kept in the core of each cell, known as the “nucleus.” Individual cells each play their own roles, with proteins synthesized based on the instructions stored in their genes, and this process is how human bodies keep healthy.
What is a Genome?
Genes are made of extremely long strands of DNA. “Genome” refers collectively to the DNA strands that contain genes, and all of the genetic information inside.
The Source of Cancer
When cells divide, their genes are copied, or “replicated,” so the same genome data is found within every one of a person’s cells. Sometimes, gene replication doesn’t go perfectly, which can give rise to changes or errors in the genes, known as “mutations.” As these mutations accumulate, this can lead to cancer. However, while these mutations can lead to cancer, they are not passed on to the next generation.
Molecularly Targeted Drugs
We now know that cancer cells grow and multiply due to various protein abnormalities. These abnormalities are caused by genetic mutations. Molecularly targeted drugs focus on specific molecules, preventing the growth of cancer cells, or even destroying them. Compared to conventional anti-cancer drugs, which harm not only cancer cells, but also normal, healthy cells, molecularly targeted drugs can attack and suppress the growth of cancer cells specifically, by affecting only the molecules that are involved in the growth of cancer cells.
Rare Cancer
“Rare cancer” refers to cancers that occur in fewer than 6 out of 100,000 people per year. Because of the difficulty of definitive diagnosis and the lack of development of therapeutic agents to treat rare cancers, treatments for them have low satisfaction levels, and these cancers tend to be very severe and urgent. The Rare Cancer Center section of the National Cancer Center website has more information on rare cancers.
(Rare Cancer Center, National Cancer Center:https://www.ncc.go.jp/en/ncch/specialist_ctr_depts/Rare_Cancer_Center/index.html)
Cancer of Unknown Primary
“Cancer of unknown primary” refers to cases where it is unclear which organ the cancer originated in, even after proper tests, and where only the metastatic lesions have grown. About 1–5% of all cases of cancer are of unknown primary. These cancers are troublesome to fully cure: because of the difficulty of determining the organ that the cancer cells originate from, these cancers cannot be completely removed surgically.
Patient categories:
Cancer is a disease caused by genetic mutations, but the genetic mutations that occur in cancer cells will vary from patient to patient, even if they occur in the same organ. The genetic tests that are currently in routine use can only examine a portion of the mutations that occur in specific genes; a gene panel test, on the other hand, uses a patient’s cancer tissue and blood to comprehensively analyze anywhere from tens to hundreds of genetic mutations. The information on genetic mutations that the test provides can be used to help diagnose the cancer and choose a therapy.
For cancers of unknown primary or rare cancers, it can be difficult to choose a therapy; however, if a gene panel test can be used to infer which genetic mutations are causing the cancer, it may be possible to treat the mutations directly through molecularly targeted drugs. Even if a patient does not respond to a standard treatment, a gene panel test may provide information (including clinical research) about which drugs would be expected to be effective against the genetic mutations in the cancer cells. However, it is also possible that the test may not provide any information that would be useful for the diagnosis or treatment of the patient's cancer.
Gene panel testing can reveal genetic mutations in a patient’s cancer tissue, and provide information on drugs and clinical studies expected to be effective against these mutations. The information obtained may include drugs that have not been approved for use in Japan, or clinical studies that have not yet been conducted in Japan.
This test detects cancer-related genes (somatic genes). However, there is a possibility of finding genetic mutations related to hereditary diseases in a small percentage of cases, so it is important for patients to be fully informed of this possibility before the test. It is vital to demonstrate the utmost ethical care when handling information about genetic mutations related to hereditary diseases.
The samples used for gene panel tests are pathological tissue collected during surgery or through a biopsy, or patient blood. When using preserved samples, they must meet the storage standards for samples to be used.
The samples used for this test are FFPE specimen* samples that meet the following standards. Each test requires about ten unstained FFPE specimens, cut into 10 µm slices (specifics vary by type of sample).
The sample should be selected from a site that contains as much tumor as possible.
(At least 20% tumor; if possible, at least 50%)
*Formalin fixed paraffin embedded specimen:
A formalin solution is used to solidify tissue taken from surgery or a biopsy, which is then embedded in a block of paraffin (essentially melted candle wax). The block is cut into thin slices, and the slices are mounted onto glass slides. FFPE specimens can be prepared by the medical institution that performed your surgery or biopsy.
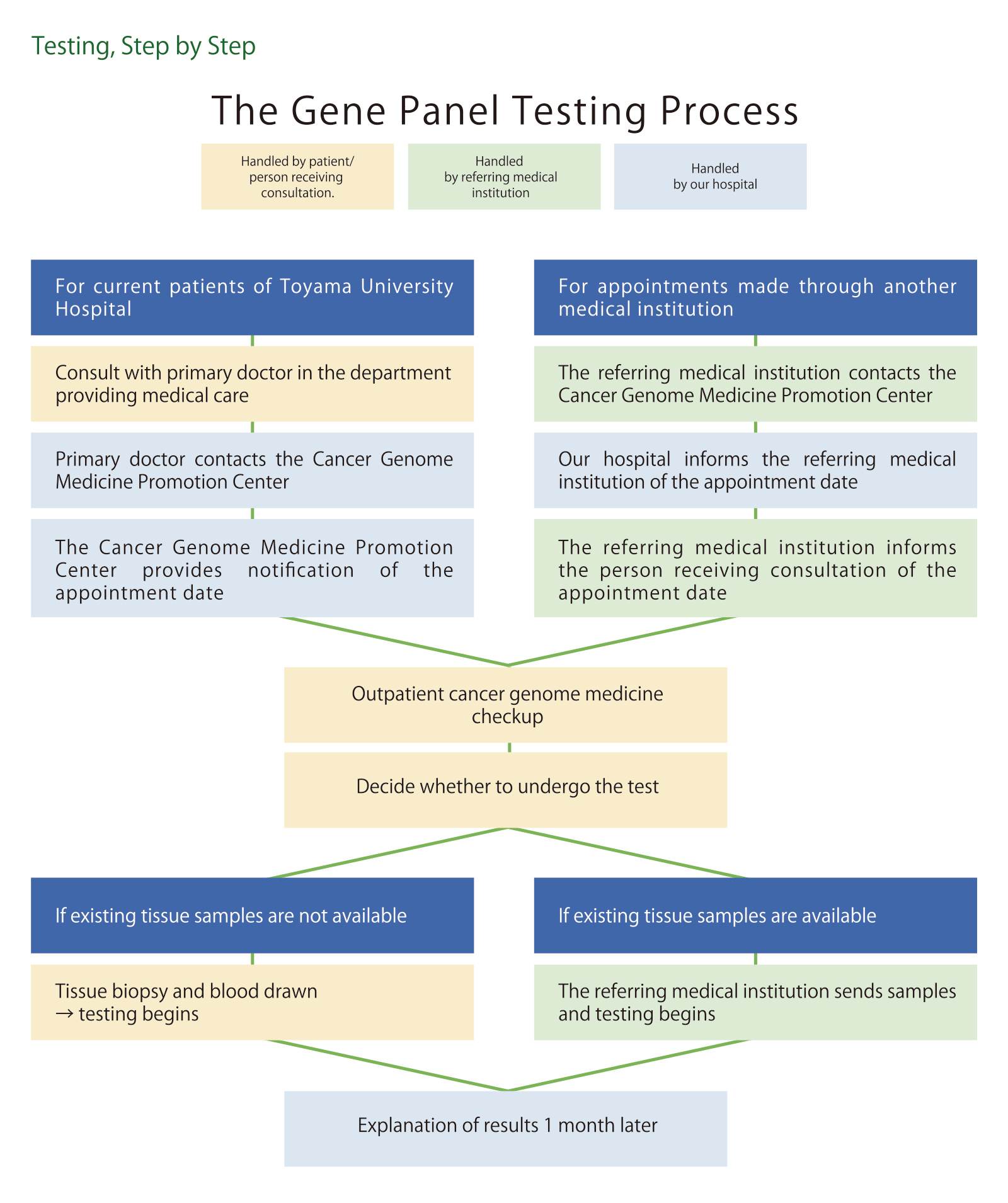
Consult your primary doctor at your current medical institution. If you are deemed eligible for cancer genome medicine, fill out the application form, and ask your primary doctor to prepare a written referral. Next, have the medical institution contact the Cancer Genome Medicine Promotion Center. The Center will provide details of the date and time of your appointment at a later date, to your medical institution, which will then contact you to let you know when you should come to the Center for your appointment.
Application Form (PDF)
Tel 076(434)7725
Fax 076(415)8862
If you are currently a patient at Toyama University Hospital, consult with your primary doctor.
Some forms of cancer genome medicine are covered by national health insurance, while others are not. The patient is responsible for the full cost of any tests not covered by national health insurance. Please note that, if the results of a gene panel test suggest the use of a drug that is not covered by national health insurance, the patient may also be responsible for the full cost of the treatment, as well.
For specific costs, see the section below.
Gene Panel Tests Available at Toyama University Hospital (PDF)
Because this test is still in development, it may produce results that are less certain compared to conventional treatments. We ask that you carefully consider each of the following precautions before undergoing this test.
Gene panel testing is an advanced technology that has developed rapidly; at the same time, however, it still retains a degree of uncertainty. For instance, the test might not provide information that would prove useful in selecting a therapy, or, if it does lead to the discovery of a candidate drug, that drug might not be available off-label (i.e. for a use other than its officially intended purpose), or the drug may still be unapproved. Additionally, the analysis itself may be unsuccessful, based on the quality of the samples used.
Because genes serve as a “blueprint” for cells, DNA sequencing can reveal a person's unique characteristics. In particular, some diseases, known as “hereditary diseases,” have been found to be defined by certain genetic sequences. If one of these is discovered, it becomes possible to consider the future development of the disease and its potential impact on blood relatives.
However, the genetic mutations in cancer cells are generally not passed from parents to children. Rather, these are what are known as “acquired mutations,” or mutations that have occurred gradually over the course of one’s life. Therefore, in most cases, there is no need to worry about hereditary diseases.
However, there are certain types of cancer, known as “familial cancer,” that are more likely to develop due to inborn gene sequences; in these cases, other considerations need to be made. You have the right to choose whether or not you would like the results disclosed to you if such a genetic mutation is found, and we will confirm your preferences using the test consent form.
On the one hand, if such a genetic mutation is found, knowing the results may prove useful for the early detection and treatment of diseases that could later develop. On the other hand, this knowledge could potentially bring worry or emotional distress to you or your family.
Some forms of this test are covered by national health insurance, while others are not. If you wish to receive treatment based on the results of this test, please first consult with the doctor in charge at the hospital where you are currently receiving treatment. The test results may suggest drugs that have not been approved for use in Japan (unapproved drugs), or that are off-label, meaning that they have been approved, but are not covered by national health insurance for use on your cancer. Treatment involving drugs such as these is not covered by national health insurance, so you may have to fully cover the high cost of treatment.
Under some circumstances, it may not be possible to prepare a test report. Depending on the condition of the sample (cancer tissue) submitted for this test, we may be unable to prepare a final report, such as if the sample is too old, or if there is advanced tissue breakdown.
If, for whatever reason, it would not be feasible to provide an explanation of your test results to you, we can instead explain your results to another person designated by you in advance (a proxy). If you wish to designate a proxy, please note that you must provide that proxy’s name and contact information on the consent form.
| OncoGuide™ FoundationOne® CDx |
The treatment counts as 56,000 national health insurance points (¥560,000), and 10–30% of the treatment cost is to be paid by the patient. |
| Cancer Genome Medicine Consultation Fees | Per hour | ¥8,800 | |
| OncoPrime | OncoPrime Cancer Genetic Test | ¥959,200 | |
| In the event of test cancellation due to the condition of the tissue sample (If genetic testing could not be properly performed due to sample condition, etc.) |
¥356,400 | ||
| Guardant360 | Guardant Liquid Biopsy Cancer Genetic Test | First Test | ¥404,800 |
| Subsequent Tests | ¥299,200 | ||
Specific Medical Fields
Advanced Breast Cancer Treatment and Breast Reconstruction Center
Pancreas and Biliary Tract Center
Pediatrics, AYA Generation, and Fertility Center
Robotic Surgery Center
Radiotherapy Center
Oncothermia Center
Blood Oncology Center
Head and Neck Oncology Center
Chest Oncology Center
Gastrointestinal Oncology Center
Urological Oncology Center
Gynecological Oncology Center
Sarcoma and Rare Cancer Center
Genetic Oncology Center
Patient Support Fields
Outpatient Chemotherapy Center
Palliative Care Center
Cancer Rehabilitation Center
Cancer Consultation and Support Center
Japanese Oriental Medicine Center
Treatment Support Fields Regimen Registry Department In-House Cancer Registry Department Human Resource Development Department Cancer Board Department Biobank Department
Advanced Medical Development Center Cancer Genome Medicine Promotion Center Cancer Immunotherapy Center
Link to "Advanced Medical Care at Toyama University Hospital